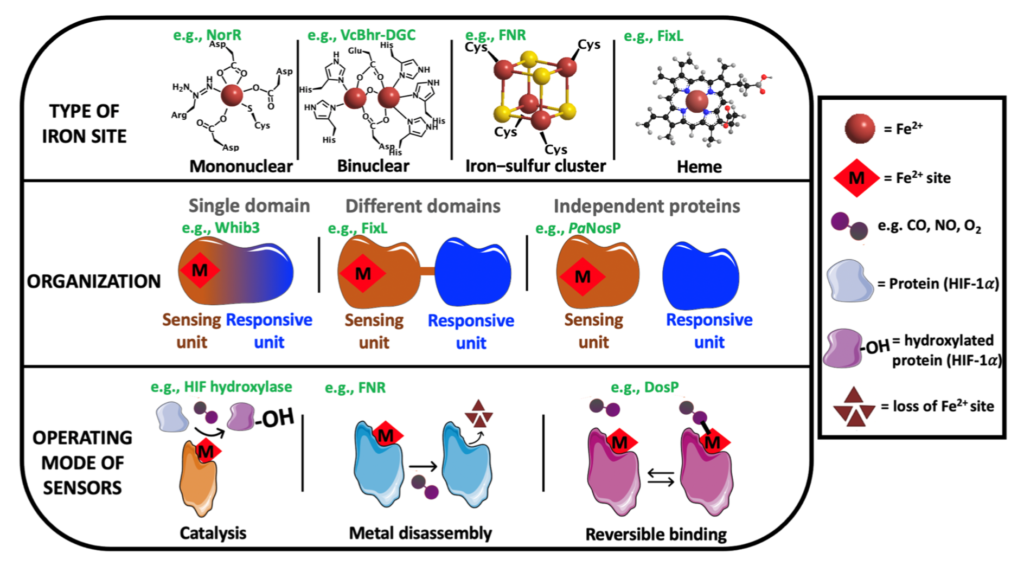
| Our studies are mainly centered on heme proteins, particularly investigating heme-based sensors such as the oxygen sensors FixL (from Rhizobia), DevS and DosT (from M. tuberculosis), among others.
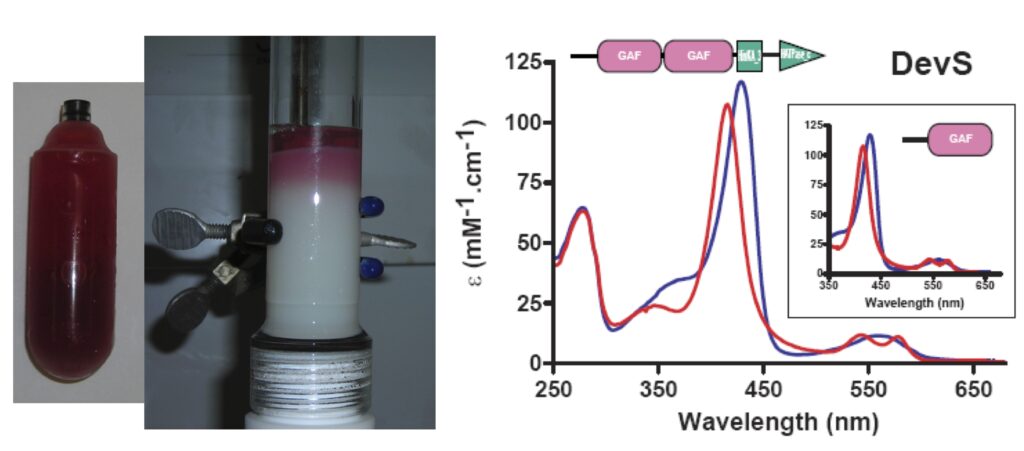
This mature class of heme proteins is dedicated to sense small gaseous molecules (e.g., CO, NO and O2), which has been found in all kingdom of nature, from humans to bacteria, exhibiting many important biological roles, such as in symbiosis for nitrogen fixation, biofilm control, chemotaxis, virulence, blood pressure control, human biological clock, among others. We prepare and isolate recombinant proteins and also make mutants to investigate their mechanism of function, exploring spectroscopic, electrochemical and chromatographic techniques.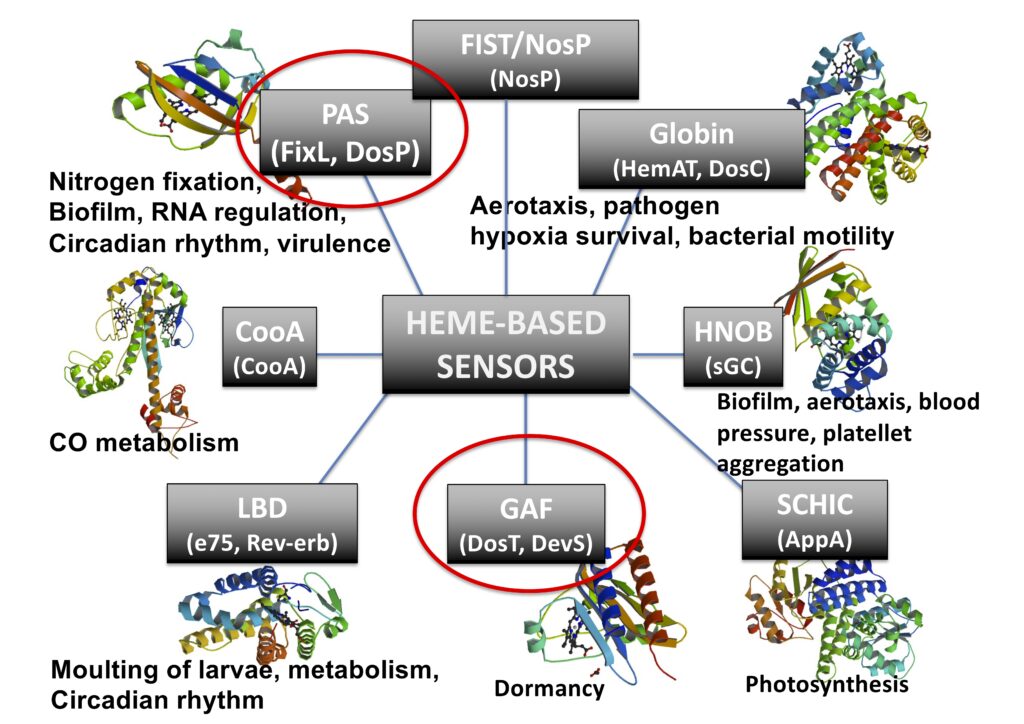
FixL has been a prototype of heme-based sensor kinase proteins and much have been learned about it. One interesting correlation of midpoint eletrochemical potential of the heme with kinase activity was proposed by our lab during studies with a hybrid FixL from R. etli.
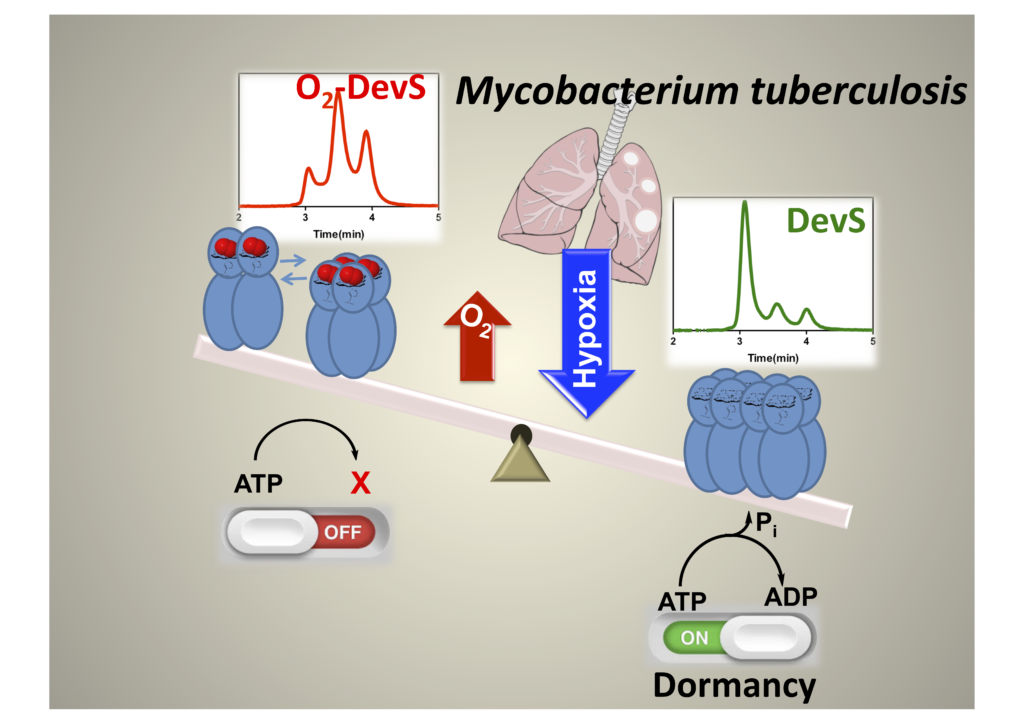
Our independent work has showed DevS from M. tuberculosis has a peculiar mechanism of signal transduction, which upon binding to oxygen the quaternary structure is mainly dimeric and tetrameric maintaining histidine kinase activity off. This changes under anaerobic conditions when octameric structures are quickly formed allowing histidine kinase to become active.
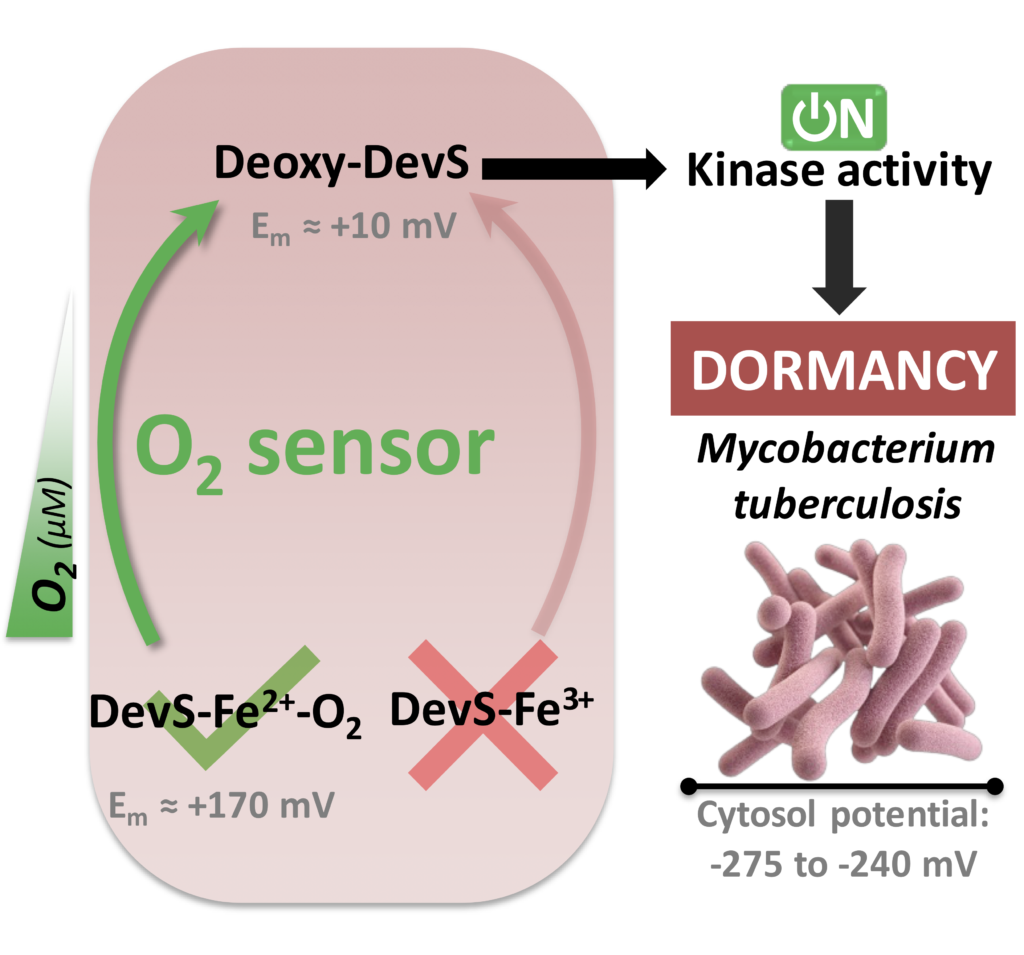
 In another work, a detailed spectroelectrochemical study of DevS showed its reasonably high potential in agreement to its role as an oxygen sensor, discarding a redox sensing activity. Additionally, a nice pH dependence was shown with even higher electrochemical potential at moderately low pH, which further supports during hypoxia (usually creating an acid environment) oxygen sensing is the main function of this sensor. In another work, a detailed spectroelectrochemical study of DevS showed its reasonably high potential in agreement to its role as an oxygen sensor, discarding a redox sensing activity. Additionally, a nice pH dependence was shown with even higher electrochemical potential at moderately low pH, which further supports during hypoxia (usually creating an acid environment) oxygen sensing is the main function of this sensor.
There are also other contributions in revisions making clearer multiple aspects of the organization and functioning of these systems and other related gas sensor metalloproteins.
 |